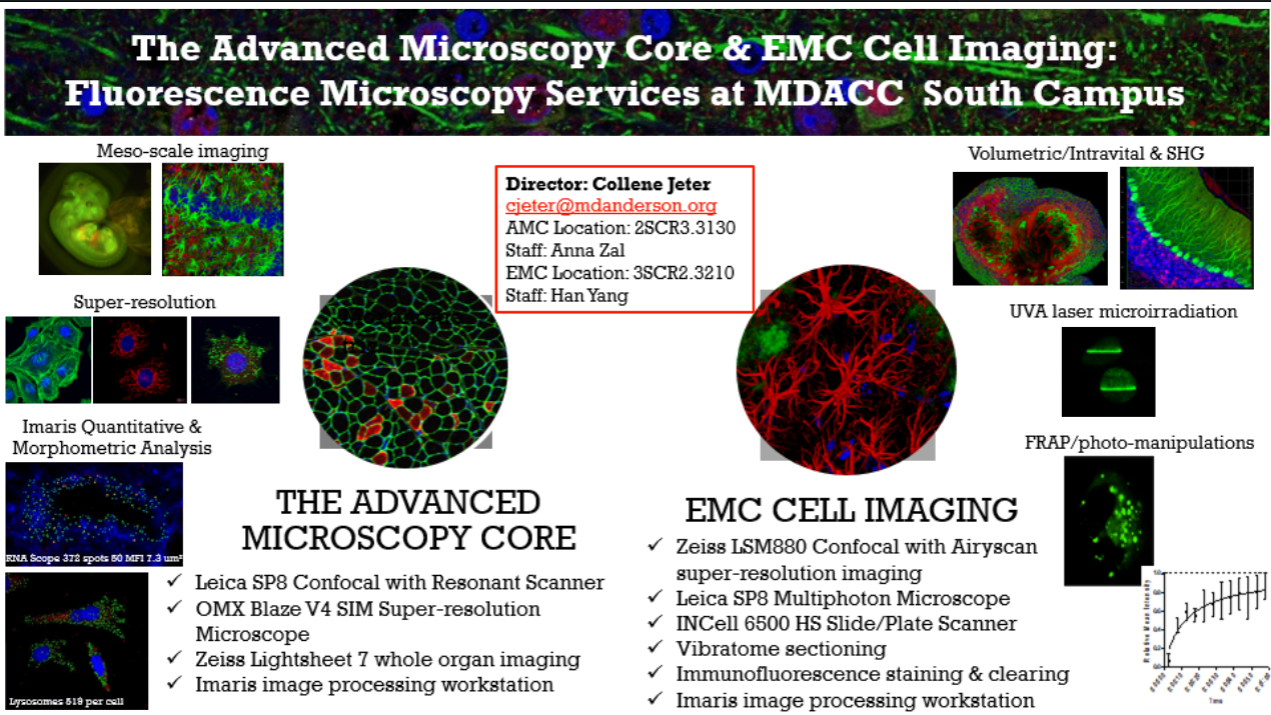
Overview of Services
Meso and Micro-Scale Lightsheet Microscopy
Equipment: Zeiss Gaussian Lightsheet 7 (LS7) | LifeCanvas Smart SPIM Lightsheet
- Rapid volumetric imaging of fixed and cleared whole mouse and rat organs, tumor fragments and embryos
- Dual sided lightsheet illumination
- LS7 lasers: 405, 445, 488, 514, 561 and 638 nm
- LifeCanvas lasers: 445, 488, 561 and 633 nm
Laser Scanning Confocal Microscopy with Spectral HyD Detectors
Equipment: Leica SP8 Confocal Microscope | Zeiss LSM880 Confocal Microscope
- Fast imaging of live or fixed cells and tissues by resonant or conventional scanning
- Highest sensitivity photon-counting HyD detectors
- Spatial resolution ~250 nm (XY), ~600 nm (Z)
- Channel unmixing by spectral fingerprinting
- 3-D visualization
- Reflection mode using AOBS
- FRET and FRAP modules
- Automated image stitching for large specimens and 3-D Assay imaging in multi-well plates
- Inverted configuration
Intravital Multiphoton (2-photon) Microscopy
Equipment: Leica SP8 DIVE Multiphoton Microscope
- Dynamic imaging of cellular behavior in vivo
- Immune surveillance
- Cancer metastasis and invasion
- Expertise in intravital tumor microscopy including in skin, lung, brain, pancreas, liver, bone and lymph nodes
- Thick specimen imaging - fresh or fixed tissue, organoids
- Conventional galvo point scanning
- 8 kHz resonant scanning for highest speed
- Non-linear optical imaging by second and third harmonics (SHG/THG)
- SpectraPhysicsInsight X3 dual beam laser 680-1300 nm and 1040 nm
- 4 high QE non-descanned spectral hybrid (HyD) detectors
- Heated animal platform and EZ-9000 anesthesia
- Scientifica stage and incubation with CO2 control
- VueBio Organ Suction Holder
- Upright configuration
Super-Resolution Structured Illumination Microscopy (SIM)
Equipment: OMX Blaze V4 Microscope
- Spatial resolution ~120 nm (XY), ~300 nm (Z)
- 3-D high impact imaging of cellular structures
Total Internal Reflection Microscopy (TIRF)
Equipment: OMX Blaze V4 Microscope
- Spatial resolution ~250 nm (XY), ~ 100 nm (Z thickness)
- Studies of live cell membrane receptor-ligand interactions, cellular adhesion, endo and exocytosis, etc.
Fluorescence Recovery After Photobleaching (FRAP)
Equipment: Leica SP8 Confocal Microscope | Zeiss LSM880 Confocal Microscope
- Dynamics of molecule trafficking in cells
Super-Resolution Localization Microscopy (STORM/PALM/GSD)
Equipment: OMX Blaze V4 Microscope (2-D)
- Spatial resolution ~20 nm (XY), ~ 60 nm (Z)
- Molecular studies of subcellular structures at the highest optical resolution available
Förster/Fluorescence Resonance Energy Transfer (FRET) Imaging
Equipment: OMX Blaze V4 Microscope | Leica SP8 Confocal Microscope | Zeiss LSM880 Confocal Microscope
- 1-10 nm molecular distance sensitivity
- Static and dynamic mapping of molecular interactions inside cells
- ‘Cyan/Yellow’, ‘Green/Red’ and ‘Red/Far Red’ donor/acceptor compatibility
- Sensitized Emission (3-Cube) Method or Photobleaching Method
Image Analysis and Visualization
IMARIS Software (2SCR3.3203 and 3SCR2.3412)
- High-end 3/4D Image Visualization and Analysis
- Adaptive thresholding with feature identification (spots, cells, nuclei, cytoplasm, cell membranes, neuronal dendrites, etc)
- Hundreds of morphological measurements
- Colocalization
- 3-D video design with rotations, fly-through, zooming and time-lapse visualization
- Spatiotemporal tracking
- Compatible with most existing microscope brands and image formats
LAS X (Leica Confocal Software) (2SCR3.3130)
- Image pre-processing and basic analysis of images from Leica SP8 Confocal Microscope
- De-noising, spectral channel unmixing, distance measurement, z-stack projection (maximum intensity or average), 3-D rotation movie rendering, colocalization coefficients
SoftWorx (OMX microscope)
- Image pre-processing and basic analysis of images from OMX Blaze SIM Microscope
- De-noising, distance measurement, z-stack projection (maximum intensity or average), 3-D rotation movie rendering, colocalization coefficients, FRET, etc.
Contact
Collene Jeter |Core Director | cjeter@mdanderson.org| 832-750-7259 (office) & 512-694-0586 (cell)
M. Anna Zal |Core Manager | mazal@mdanderson.org| 713-563-7306 (office) & 346-234-0480 (cell)
Han Yang | Sr Research Asst | hyang13@mdanderson.org | 713-794-1902 (office)
Locations and hours of operation
| Hours |
Locations |
|
Monday - Friday 8:00 am-5:00 pm (24/7 for independent users)
|
2SCR3.3023 (office) 7455 Fannin, Houston, TX 77054
3SCR4.3420 (office) 1881 East Rd., Houston, TX 77054
|
Links and Resources
- MD Anderson Advanced Microscopy Core
Search available services:
 View: by category
alphabetically
View: by category
alphabetically
| Name |
Description |
Price |
|
Confocal Leica SP8 Laser Scanning - Assisted
|
|
Inquire
|
|
Confocal Leica SP8 Laser Scanning - Independent
|
|
Inquire
|
|
Confocal Leica SP8 Laser Scanning - Time-Lapse (>8 hours)
|
Time-lapse retes are available for reservations above 8 hours.
|
Inquire
|
|
Confocal Leica SP8 Laser Scanning - Training
|
|
Inquire
|
|
Confocal Zeiss LSM880 with Airyscan - Assisted
|
Hourly rate
|
Inquire
|
|
Confocal Zeiss LSM880 with Airyscan - Independent
|
Hourly rate
|
Inquire
|
|
Confocal Zeiss LSM880 with Airyscan - Time-Lapse
|
Available weekends (3 day maximum)
Set up fee may be required
|
Inquire
|
|
Confocal Zeiss LSM880 with Airyscan - Training
|
Hourly rate
|
Inquire
|
|
Imaging Consultation
|
No charge
|
Inquire
|
|
Imaris C1 Image Analysis - Assisted
|
Hourly rate
|
Inquire
|
|
Imaris C1 Image Analysis - Independent
|
Hourly rate
|
Inquire
|
|
Imaris C1 Image Analysis - Training
|
Hourly rate
|
Inquire
|
|
Imaris Image Analysis - Assisted
|
|
Inquire
|
|
Imaris Image Analysis - Independent
|
|
Inquire
|
|
Imaris Image Analysis - Training
|
|
Inquire
|
|
INCell Analyzer 6500 HS Slide/Plate Scanner - Independent
|
Slide/Plate scanning
|
Inquire
|
|
INCell Analyzer 6500 HS Slide/Plate Scanner - Training/Assisted
|
30 min; further assistance charge at Assisted rate
|
Inquire
|
|
LifeCanvas Active Clearing
|
Detergent-based and electrophoretically enhanced whole organ clearing
Consultation with core staff prior to sample preservation/submission required *SHIELD preservation REQUIRED
May not be compatible with all tissue/organ types and/or epitopes
|
Inquire
|
|
LifeCanvas Active IF Staining
|
Electrophoretically enhanced whole organ IF staining
Compatible with LifeCanvas validated antibodies; additional may be tested (see core staff)
*Antibodies not provided as part of service fee
Consultation with core staff prior to sample preservation/submission required
May not be compatible with all tissue/organ types and/or epitopes
|
Inquire
|
|
LifeCanvas Passive Tissue Clearing
|
Detergent-based tissue clearing
Consultation with core staff prior to sample preservation/submission required *SHIELD preservation recommended
May not be compatible with all tissue/organ types and/or epitopes
|
Inquire
|
|
Lightsheet Zeiss LS7 - Training
|
|
Inquire
|
|
Lightsheet Zeiss LS7 - Assisted
|
|
Inquire
|
|
Lightsheet Zeiss LS7 - Independent
|
|
Inquire
|
|
Lightsheet Zeiss LS7 - Overnight
|
|
Inquire
|
|
Multiphoton Leica TCS SP8 DIVE - Independent
|
Requires prior TCS SP8 training and core management approval
Can be added to assisted rate following sample set-up for long sample runs
|
Inquire
|
|
Multiphoton Leica TCS SP8 DIVE - Intravital Setup
|
|
Inquire
|
|
Multiphoton Leica TCS SP8 DIVE - Training/Assisted
|
With core management approval
4 hr miminum training; further assistance charged at Assisted rate
|
Inquire
|
|
Primary Antibody/Dye
|
Per sample/coverslip
|
Inquire
|
|
Standard Immunofluorescence Staining
|
Per slide
Core provided primary antibody/dye charged separately
Additional fee for FFPE processing
|
Inquire
|
|
Standard New Antibody Development
|
6 sample maximum
Confocal imaging charged separately as needed
|
Inquire
|
|
Super-Resolution OMX Blaze V4 - Independent
|
|
Inquire
|
|
Super-Resolution OMX Blaze V4 - Training/Assisted
|
|
Inquire
|
|
Thick Tissue New Antibody Development
|
6 sample maximum
Multiphoton imaging charged separately
|
Inquire
|
|
Thick Tissue Staining
|
Core provided primary antibody/dye charged separately
|
Inquire
|
|
Vibratome Sectioning - Training/Assisted
|
Per hour
|
Inquire
|
|
Vibratome Sectioning - Unassisted
|
Per sample; 10 sections per sample maximum
Subject to availability/approval
|
Inquire
|
|
Zeiss Axio Observer Z1 - Independent
|
Hourly rate; <30 min = no charge
|
Inquire
|
|
Zeiss Axio Observer Z1 - Time-Lapse (>8 hours)
|
Hourly rate (12 h max rate per day)
Set up fee may be required
Burner fee required for fluorescence imaging
|
Inquire
|
|
Zeiss Axio Observer Z1 - Training/Assisted
|
30 min; further assistance charged at Assisted rate
|
Inquire
|
|
▼
►
Confocal LSM880 (4)
|
| Name |
Description |
Price |
|
Confocal Zeiss LSM880 with Airyscan - Assisted
|
Hourly rate
|
Inquire
|
|
Confocal Zeiss LSM880 with Airyscan - Independent
|
Hourly rate
|
Inquire
|
|
Confocal Zeiss LSM880 with Airyscan - Time-Lapse
|
Available weekends (3 day maximum)
Set up fee may be required
|
Inquire
|
|
Confocal Zeiss LSM880 with Airyscan - Training
|
Hourly rate
|
Inquire
|
|
▼
►
Confocal Leica SP8 (4)
|
| Name |
Description |
Price |
|
Confocal Leica SP8 Laser Scanning - Assisted
|
|
Inquire
|
|
Confocal Leica SP8 Laser Scanning - Independent
|
|
Inquire
|
|
Confocal Leica SP8 Laser Scanning - Time-Lapse (>8 hours)
|
Time-lapse retes are available for reservations above 8 hours.
|
Inquire
|
|
Confocal Leica SP8 Laser Scanning - Training
|
|
Inquire
|
|
▼
►
Imaris (6)
|
| Name |
Description |
Price |
|
Imaris Image Analysis - Assisted
|
|
Inquire
|
|
Imaris Image Analysis - Independent
|
|
Inquire
|
|
Imaris Image Analysis - Training
|
|
Inquire
|
|
Imaris C1 Image Analysis - Assisted
|
Hourly rate
|
Inquire
|
|
Imaris C1 Image Analysis - Independent
|
Hourly rate
|
Inquire
|
|
Imaris C1 Image Analysis - Training
|
Hourly rate
|
Inquire
|
|
▼
►
Lightsheet LS7 (4)
|
| Name |
Description |
Price |
|
Lightsheet Zeiss LS7 - Training
|
|
Inquire
|
|
Lightsheet Zeiss LS7 - Assisted
|
|
Inquire
|
|
Lightsheet Zeiss LS7 - Independent
|
|
Inquire
|
|
Lightsheet Zeiss LS7 - Overnight
|
|
Inquire
|
|
▼
►
Multiphoton (3)
|
| Name |
Description |
Price |
|
Multiphoton Leica TCS SP8 DIVE - Intravital Setup
|
|
Inquire
|
|
Multiphoton Leica TCS SP8 DIVE - Training/Assisted
|
With core management approval
4 hr miminum training; further assistance charged at Assisted rate
|
Inquire
|
|
Multiphoton Leica TCS SP8 DIVE - Independent
|
Requires prior TCS SP8 training and core management approval
Can be added to assisted rate following sample set-up for long sample runs
|
Inquire
|
|
▼
►
Super resolution OMX (2)
|
| Name |
Description |
Price |
|
Super-Resolution OMX Blaze V4 - Independent
|
|
Inquire
|
|
Super-Resolution OMX Blaze V4 - Training/Assisted
|
|
Inquire
|
|
▼
►
Wet Lab (10)
|
| Name |
Description |
Price |
|
LifeCanvas Active Clearing
|
Detergent-based and electrophoretically enhanced whole organ clearing
Consultation with core staff prior to sample preservation/submission required *SHIELD preservation REQUIRED
May not be compatible with all tissue/organ types and/or epitopes
|
Inquire
|
|
LifeCanvas Active IF Staining
|
Electrophoretically enhanced whole organ IF staining
Compatible with LifeCanvas validated antibodies; additional may be tested (see core staff)
*Antibodies not provided as part of service fee
Consultation with core staff prior to sample preservation/submission required
May not be compatible with all tissue/organ types and/or epitopes
|
Inquire
|
|
LifeCanvas Passive Tissue Clearing
|
Detergent-based tissue clearing
Consultation with core staff prior to sample preservation/submission required *SHIELD preservation recommended
May not be compatible with all tissue/organ types and/or epitopes
|
Inquire
|
|
Primary Antibody/Dye
|
Per sample/coverslip
|
Inquire
|
|
Standard Immunofluorescence Staining
|
Per slide
Core provided primary antibody/dye charged separately
Additional fee for FFPE processing
|
Inquire
|
|
Standard New Antibody Development
|
6 sample maximum
Confocal imaging charged separately as needed
|
Inquire
|
|
Thick Tissue New Antibody Development
|
6 sample maximum
Multiphoton imaging charged separately
|
Inquire
|
|
Thick Tissue Staining
|
Core provided primary antibody/dye charged separately
|
Inquire
|
|
Vibratome Sectioning - Unassisted
|
Per sample; 10 sections per sample maximum
Subject to availability/approval
|
Inquire
|
|
Vibratome Sectioning - Training/Assisted
|
Per hour
|
Inquire
|
|
▼
►
unclassified (6)
|
| Name |
Description |
Price |
|
Zeiss Axio Observer Z1 - Independent
|
Hourly rate; <30 min = no charge
|
Inquire
|
|
Zeiss Axio Observer Z1 - Time-Lapse (>8 hours)
|
Hourly rate (12 h max rate per day)
Set up fee may be required
Burner fee required for fluorescence imaging
|
Inquire
|
|
Zeiss Axio Observer Z1 - Training/Assisted
|
30 min; further assistance charged at Assisted rate
|
Inquire
|
|
Imaging Consultation
|
No charge
|
Inquire
|
|
INCell Analyzer 6500 HS Slide/Plate Scanner - Training/Assisted
|
30 min; further assistance charge at Assisted rate
|
Inquire
|
|
INCell Analyzer 6500 HS Slide/Plate Scanner - Independent
|
Slide/Plate scanning
|
Inquire
|
 ALERT
ALERT 